A large study shows that even brief, irregular walking patterns, such as a few active days a week, can meaningfully lower the risk of death and heart disease in older women, challenging the 10,000-step myth.
Study: Association…

A large study shows that even brief, irregular walking patterns, such as a few active days a week, can meaningfully lower the risk of death and heart disease in older women, challenging the 10,000-step myth.
Study: Association…
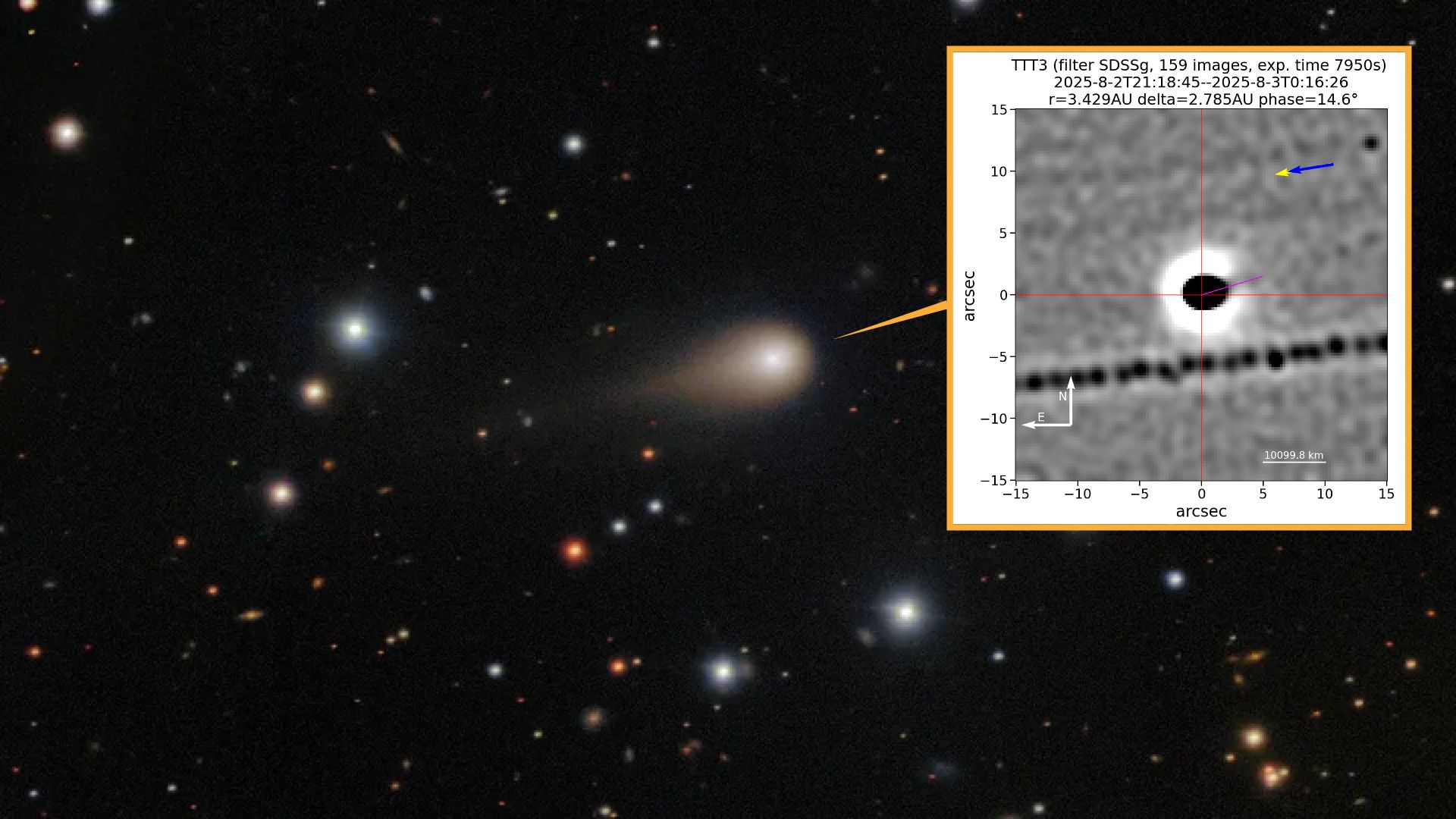
Newly released images of the interstellar comet 3I/ATLAS appear to show the alien object spitting out an enormous jet of gas and dust toward the sun — just as comets are expected to do.
Discovered in late June and confirmed by NASA in early…

Researchers have identified the largest known impact crater on Earth formed…

Netflix’s Black Rabbit took over the No. 1 spot on Nielsen’s streaming charts in its second week of release.
The drama starring Jude Law and Jason Bateman delivered 1.26 billion minutes of viewing for the week of…

American Ballet Theater doesn’t typically align its seasonal galas with a company member’s farewell, but in the case of Misty Copeland, whose trailblazing career has irrevocably changed the 85-year-old institution, an exception was made. A…

The FDA has approved belantamab mafodotin-blmf (Blenrep) in combination with bortezomib (Velcade) and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least 2 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMID).1
The approval is supported by findings from the phase 3 DREAMM-7 (NCT04246047) trial. In the study, among patients who had received at least 2 prior lines of therapy, including a PI and an IMID, BVd elicited a 51% reduction in the risk of death (HR, 0.49; 95% CI, 0.32-0.76) compared with DVd. Treatment with the combination also led to a median progression-free survival (PFS) of 31.3 months (95% CI, 23.5-NR) vs 10.4 months (95% CI, 7.0-13.4) with DVd (HR, 0.31; 95% CI, 0.21-0.47). The safety and tolerability profiles of DVd and BVd were consistent with the known safety profiles of the individual agents, according to the news release.
“With the approval of [belantamab mafodotin], we now have a community-accessible BCMA-targeting agent with the potential to improve outcomes for patients following 2 or more prior lines of treatment, where options are limited,” Sagar Lonial, MD, chief medical officer and chair of the Emory Department of Hematology and Medical Oncology at the Winship Cancer Institute of Emory University in Atlanta, Georgia, stated in a news release “This approval marks an important advance in the US relapsed/refractory treatment landscape.”
This approval follows a divided
“Today’s FDA approval of [belantamab mafodotin] is another significant milestone, providing potential for superior efficacy, including overall survival, to US patients,” Tony Wood, chief scientific officer of GSK, added in the news release.1 “There is an urgent need for new and novel therapies, as nearly all patients with multiple myeloma experience relapse, and re-treating with the same mechanism of action often leads to suboptimal outcomes. As the only anti-BCMA agent that can be administered across health care settings, including in community centers where 70% of patients receive care, [belantamab mafodotin] fulfills a major patient need. We believe [belantamab mafodotin] can redefine treatment for patients with multiple myeloma in all parts of the world, and we are accelerating its development in earlier lines of therapy to support its use across all stages of this difficult-to-treat cancer.”
In the open-label DREAMM-7 trial, patients with relapsed/refractory multiple myeloma received either BVd or daratumumab (Darzalex) plus bortezomib and dexamethasone (DVd).3 Eligible patients had received at least 1 prior therapy but had not previously received a BCMA-targeted agent and were not refractory to anti-CD38 therapy.
Grade 3 or higher adverse effects (AEs) were more frequent in the BVd arm (95%) than in the DVd arm (78%). Ocular toxicity was more common in patients receiving belantamab mafodotin, occurring at a rate of 79% vs 29% in the DVd arm, but was largely reversible with dose modifications.
“The reality for most patients with multiple myeloma is a relentless cycle of remission and relapse, as their disease becomes refractory to treatments,” Michael Andreini, president and chief executive officer of the Multiple Myeloma Research Foundation and the Multiple Myeloma Research Consortium, added in the news release. “Patients urgently need more effective treatment options that can offer more quality time with their loved ones. We see the potential for [belantamab mafodotin] in combination to help patients achieve this.”
From 12-18 October 2025, the United Nations Office for Disarmament Affairs (UNODA) and the United Nations Department of Safety and Security (UNDSS) organized the fifth training course on ‘Safe and Secure Approaches in Field Environments’…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

The company, one of the largest U.S. makers of semiconductor manufacturing equipment, will take a charge of $160 million to $180 million for the layoffs, mostly in the fourth quarter of fiscal 2025, it disclosed in a regulatory filing.
Sign up here.
Late in September, Washington cracked down on companies in China and other countries that use subsidiaries or other foreign affiliates to circumvent export curbs on chipmaking equipment and other goods and technology.
That has made it harder for Applied Materials and its rivals to export some products and supply specific parts and services to select China-based customers without a license. The company earlier this month forecast a $600-million hit to its fiscal 2026 revenue due to the expanded curbs.
It said it started informing its employees about the layoffs earlier on Thursday. It had 35,700 full-time staff as of October 27, 2024, according to its annual report.
“Our goal is to continue to transform how we work, move faster, simplify decision-making, and focus on what matters most as we prepare Applied Materials for significant growth in the coming years,” CEO Gary Dickerson said in a memo to employees.
Reporting by Aditya Soni in Bengaluru; Editing by Alan Barona
Our Standards: The Thomson Reuters Trust Principles.